Viral Vectors & Plasmid DNA Manufacturing Market Overview
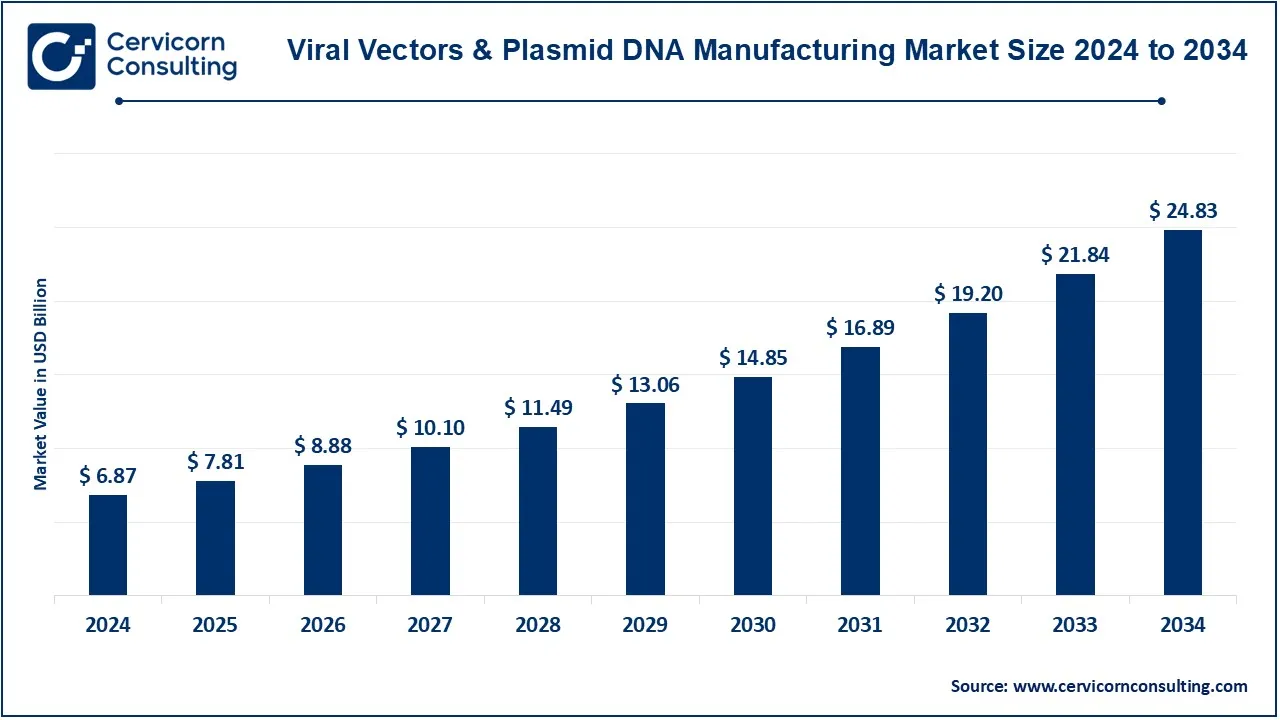
The global viral vectors and plasmid DNA manufacturing market was estimated at USD 6.87 billion in 2024 and is expected to reach around USD 24.83 billion by 2034, growing at an impressive CAGR of 20.16% from 2025 to 2034. This growth is primarily driven by the expanding use of viral vectors and plasmid DNA in gene therapy, cell-based therapies, and mRNA vaccines. Pharmaceutical and biotechnology companies are increasingly investing in scalable, high-quality production facilities to meet rising clinical and commercial demand. Government support, faster regulatory approvals for gene therapies, and an increasing number of clinical trials have further accelerated market expansion.
Get a Free Sample: https://www.cervicornconsulting.com/sample/2500
Key Market Trends
-
Advancements in Bioprocessing and Automation: Industry players are integrating AI-driven bioprocessing, automated workflows, and single-use systems to optimize production efficiency and lower costs. Single-use bioreactors, in particular, are gaining popularity due to their flexibility and minimized contamination risk.
-
Rise of mRNA Vaccines: The COVID-19 pandemic highlighted the critical need for high-quality plasmid DNA for mRNA vaccine development, resulting in a surge of over 200% in demand. This has encouraged significant investments in plasmid manufacturing infrastructure.
-
Expansion of Gene Therapy Pipelines: Viral vectors are essential in over 70% of ongoing gene therapy clinical trials, with more than 1,000 trials active worldwide. AAV vectors are widely preferred for their safety and low immunogenicity.
-
Regulatory Support and Funding: Accelerated approvals from regulatory bodies such as the FDA and EMA, alongside government-backed funding programs across North America, Europe, and Asia, have created an enabling environment for therapy development.
-
Outsourcing to CDMOs: Biopharmaceutical companies increasingly rely on contract development and manufacturing organizations (CDMOs) for specialized expertise and production capacity, enabling them to concentrate on R&D.
Market Drivers
-
Growing Demand for Advanced Therapies: Increasing prevalence of cancer, genetic disorders, and infectious diseases drives the need for innovative treatments.
-
Technological Innovations: Advances in viral vector design, plasmid DNA production, and high-yield bioprocessing improve scalability and efficiency.
-
Government Initiatives: Policy support and funding for gene therapy and biomanufacturing accelerate adoption and commercialization.
-
Rising Clinical Trials: With nearly 2,900 active global gene therapy trials, robust manufacturing capacity is in high demand.
-
Commercialization of Gene Therapies: Approval of therapies such as Zolgensma and Luxturna demonstrates the commercial potential of gene therapies, further boosting market growth.
Impact of Trends and Drivers
These trends and drivers are influencing different regions and applications. North America leads the market, accounting for 48.7% of revenue in 2024, thanks to advanced infrastructure and key CDMOs. Europe and Asia-Pacific are becoming important hubs for viral vector production, supported by government initiatives and collaborations between academia and industry. Vaccinology and cancer therapies are the leading application segments, holding revenue shares of 21% and 37.7%, respectively.
Challenges & Opportunities
-
Challenges: High capital investment for GMP-compliant facilities (USD 100–250 million), complex regulatory requirements, and plasmid DNA production timelines of 6–9 months.
-
Opportunities: Growth in cell and gene therapy pipelines, increased outsourcing to CDMOs, adoption of automation, and innovations in viral vector design and high-yield manufacturing processes.
Future Outlook
The market is expected to continue its strong growth trajectory, supported by AI integration, digitalization, and scalable bioprocessing. Emerging trends such as next-generation viral vectors, advanced plasmid DNA platforms, and global expansion of CDMO networks are poised to unlock new opportunities, solidifying the market’s role in precision medicine, gene therapy, and vaccine development.
Contact Us for a Detailed Overview: https://www.cervicornconsulting.com/contact-us