In Vitro Diagnostics (IVD) Market Overview
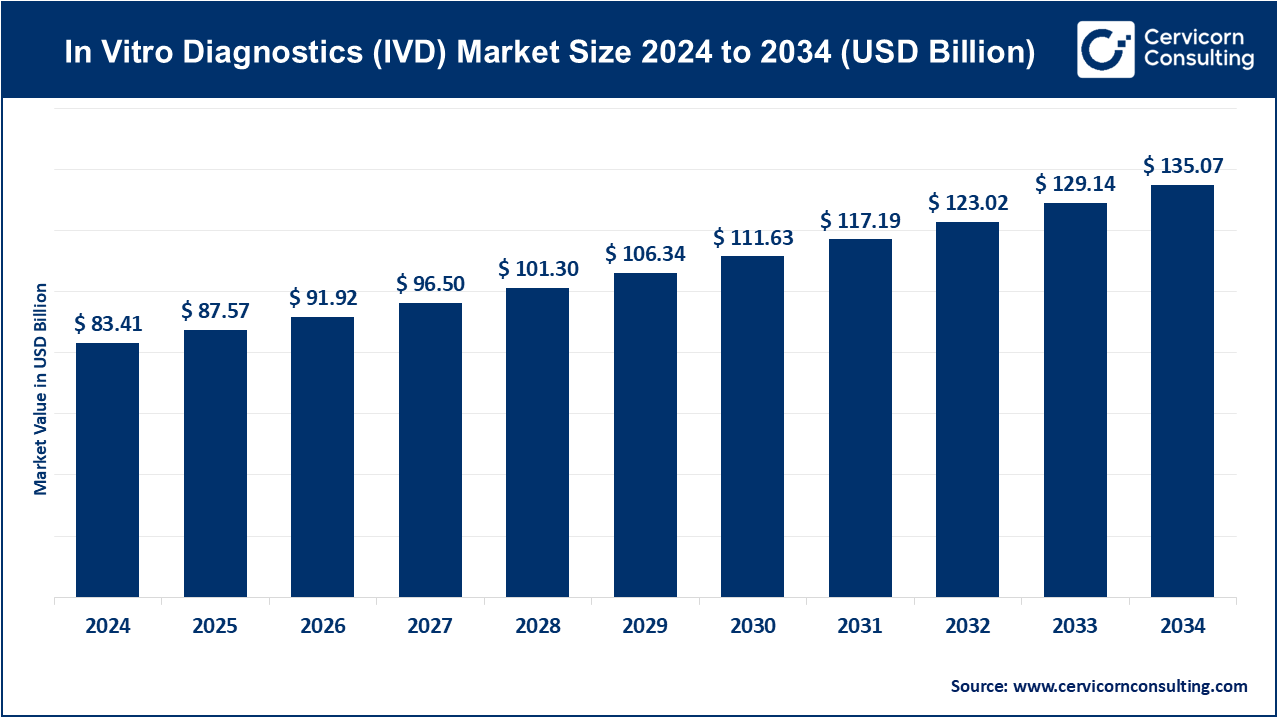
The global In Vitro Diagnostics (IVD) market is a critical component of modern healthcare, encompassing medical instruments, reagents, and technologies used to examine biological samples such as blood, urine, and tissue outside the human body. These diagnostic tools are essential for disease detection, monitoring, and prevention, providing healthcare professionals with valuable insights for clinical decision-making. The market was valued at around USD 83.41 billion in 2024 and is projected to grow to approximately USD 135.07 billion by 2034, representing a CAGR of 4.97%. This growth reflects a rising global demand for rapid, accurate, and cost-efficient diagnostic solutions.
Key Market Trends
-
Expansion of Point-of-Care Testing (POCT): Portable analyzers and rapid diagnostic kits enable testing directly at patient sites, cutting turnaround times and allowing immediate medical decisions. Examples include Abbott’s ID NOW and Roche’s cobas Liat System, which are widely used for infectious disease detection.
-
Advances in Molecular Diagnostics: Innovations such as PCR, next-generation sequencing (NGS), and CRISPR-based assays are transforming genetic and infectious disease testing. These technologies facilitate early detection of conditions like cancer, HIV, and tuberculosis, supporting personalized medicine initiatives.
-
Artificial Intelligence (AI) and Big Data Integration: AI-driven diagnostic analytics improve accuracy by interpreting complex datasets, predicting disease outcomes, and providing actionable clinical insights. This trend is particularly impactful in oncology and genomics, where large-scale data analysis is essential.
-
Laboratory Automation and Robotics: Automated analyzers, high-throughput platforms, and liquid handling systems enhance laboratory efficiency, reduce human error, and optimize resource use.
-
Evolving Regulatory Landscape: Frameworks like the EU In Vitro Diagnostic Regulation (IVDR) and fast-track FDA approvals in the U.S. ensure product quality, safety, and innovation, shaping market adoption and development.
👉 Get a Free Sample: https://www.cervicornconsulting.com/sample/2353
Market Drivers
-
Rising Prevalence of Chronic and Infectious Diseases: Increasing rates of diabetes, cardiovascular conditions, cancer, and infectious diseases such as COVID-19 have heightened the need for timely diagnostics. For instance, the pandemic caused a surge in rapid antigen and PCR testing.
-
Technological Advancements: Continuous innovation in molecular diagnostics, POCT, and laboratory automation enhances accuracy, reduces testing times, and expands diagnostic capabilities, promoting global adoption.
-
Growing Aging Population: The rising global geriatric population, more prone to chronic illnesses, has increased demand for routine and advanced diagnostic tests.
-
Government Initiatives and Healthcare Investments: Policies promoting preventive care, reimbursement schemes, and investments in healthcare infrastructure support market growth. Examples include the U.S. FDA’s accelerated approval programs and China’s healthcare modernization projects.
-
Consumer Awareness and Preventive Health Focus: Heightened health consciousness and interest in early detection have boosted demand for home-testing kits and POCT solutions, especially in urban regions.
Impact of Trends and Drivers
-
Application Segmentation: Oncology, cardiology, infectious diseases, endocrinology, and genetic testing benefit from molecular diagnostics and AI-driven solutions.
-
Regional Influence: Adoption in North America and Europe is accelerated by infrastructure and regulatory support, while Asia-Pacific growth is driven by increased healthcare spending and government initiatives.
-
Product Uptake: Molecular diagnostics, immunoassays, and POCT solutions are witnessing strong adoption due to their accuracy, speed, and convenience.
Challenges & Opportunities
-
Challenges: High costs of advanced diagnostic tools, stringent regulatory requirements, and limited infrastructure in emerging markets may impede growth.
-
Opportunities: Integration of AI, expansion of digital and home-testing solutions, and rising demand for precision medicine present significant growth potential.
Future Outlook
The IVD market is expected to continue expanding, driven by emerging trends such as AI-enabled diagnostics, advanced molecular testing, and remote POCT solutions. Increasing emphasis on preventive healthcare, rapid disease detection, and supportive government initiatives will sustain innovation and adoption across all regions.
👉 Contact for Detailed Market Insights: https://www.cervicornconsulting.com/contact-us